Effect of flux
| Effect | Remarks |
|---|---|
| Preventing oxidization | The generated oxidants are melted rapidly and the surface of the molten metal is covered uniformly in fluid slag. Moreover, there is a reducing atmosphere due to the low-moisture coating material made of low S instead of charcoal. |
| Shortening melting time | Scrap covered in oxidants takes a long time to melt. We apply a method of melting surface oxidants using flux in order to shorten melting time. |
| Reducing metal content in slag | By generating slag with fluidity, we can reduce the amount of ground metal elements in slag. |
| Alleviating adhesion of slag to furnace walls | Additional elements cause slag to adhere to furnace walls, however by using flux, we melt the slag on the furnace wall. The flux then permeates the furnace wall and it becomes easier to remove slag. |
| Removing impurities | Impurities in alloy are removed as a priority through metallurgic methods. |
| Gas removal benefits | The concentration of hydrogen, which causes blowholes, is reduced. |
Effects and precautions of
flux to reduce oxidants!
A point to be aware of regarding using flux to reduce oxidants is that, while it will reduce the oxidants during melting, in most cases, it also produces heat resistant material, therefore this will exist alongside the oxidants.
As such, furnace walls will melt if an excess amount of flux is used. However, if flux is used wisely, the amount of slag can be minimized and it becomes difficult for slag to adhere to furnace walls, enabling stable operations.
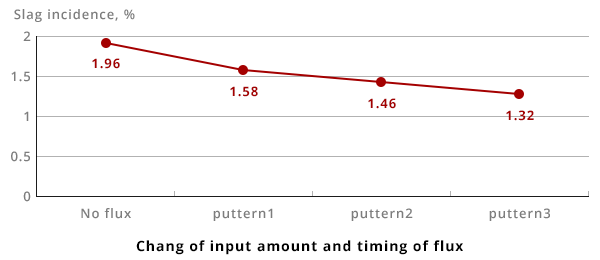
The below graph shows the amount of slag generated in brass with varying amounts of flux being used at different timing.